Polymers of amino acids, known as polypeptides or proteins, play a vital role in biological systems and have numerous applications in various fields.
Key takeaways:
- Amino acids are the building blocks of proteins.
- Amino acids polymerize through dehydration synthesis to form polypeptides.
- Peptide bonds join amino acids together in polypeptide chains.
- Polyamide polymers (nylons) have various applications in construction.
- Amino acid polymers hold potential for eco-friendly construction materials.
Definition of Amino Acids
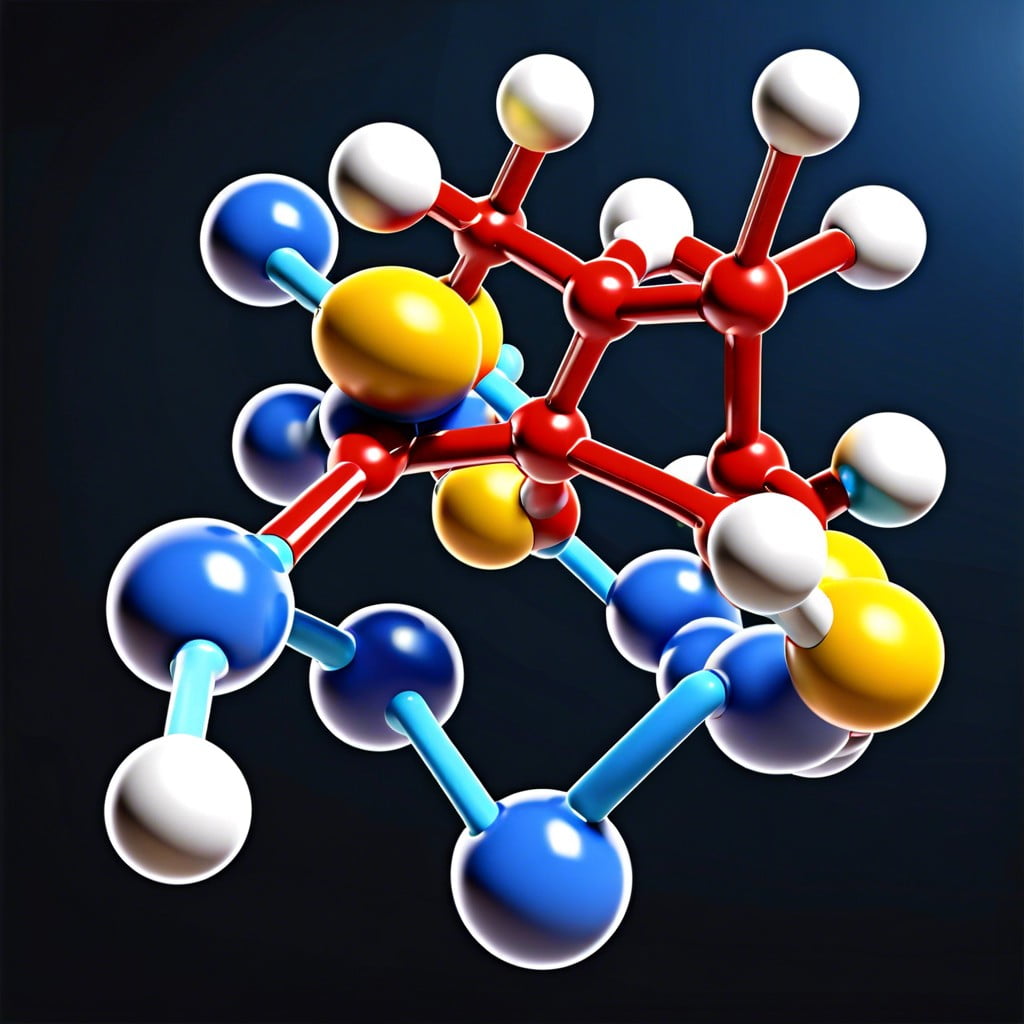
Amino acids are the building blocks of proteins, vital to all living organisms. Each amino acid consists of a central carbon atom, an amino group (NH₂), a carboxylic acid group (COOH), and a unique side chain, or R-group, that determines its properties and function.
There are 20 standard amino acids, which can link together in diverse sequences to form a vast array of proteins, each with unique functions. This linkage through a dehydration reaction leads to a chain-like structure known as a polypeptide, which then folds into a functional protein.
The specific sequence and structure of amino acids in a protein determine its characteristics and role in biological systems and materials science.
Polymerization of Amino Acids
Polymerization is a chemical reaction in which monomers link together to form long-chain molecules known as polymers. In the context of amino acids, this process occurs through dehydration synthesis, where a water molecule is removed to bond individual amino acids together.
Here are the key points about this process:
- Monomers to Polymers: Individual amino acids are the monomers that, when joined, create polypeptide chains, the polymers in this scenario.
- Dehydration Synthesis: Each bond forming between amino acids results in the release of a water molecule, hence the term “dehydration.”
- Peptide Bonds: A covalent chemical bond known as a peptide bond joins each amino acid to its neighbor.
- Sequence Matters: The order in which amino acids polymerize determines the properties of the resulting polypeptide.
- Complexity and Function: These polypeptide chains can fold into complex shapes, leading to specific functions, as seen in enzymes, hormones, and structural elements of cells.
Understanding polymerization at the amino acid level lays the groundwork for grasping how proteins, essential to both living organisms and various materials used in construction, are formed.
The Peptide Bond: Peptides and Proteins
Peptide bonds form when the amino group of one amino acid reacts with the carboxyl group of another, releasing water in a dehydration synthesis process. This bond is a type of covalent bond that is central to the structure and function of peptides, which are short chains of amino acids, and proteins, which are longer chains.
Bond Basics: Picture a line of paper dolls holding hands – that’s similar to how amino acids join via peptide bonds.
Chain Reactions: As more amino acids link together, they create a polypeptide chain which can fold into a specific three-dimensional shape necessary for the protein’s functionality.
Function Follows Form: The shape determines the protein’s role; it could be structural, like collagen, or functional, like enzymes accelerating chemical reactions.
Durability: Peptide bonds are strong, requiring significant energy to break, which contributes to the stability of proteins, a trait useful in construction materials where durability is crucial.
Understanding how peptide bonds work helps in tailoring the properties of synthetic polymers used in construction to mimic these natural, sturdy connections.
Applications of Polyamide Polymers in Construction
Polyamide polymers, commonly grouped under the term “nylons,” are used extensively in construction owing to their durability and resistance to abrasion and chemicals.
Here are specific applications that take advantage of these properties:
- Protective Coatings: Nylons are used for coatings to shield surfaces from corrosion and wear, enhancing the longevity of metal structures.
- Sealants and Adhesives: Their strong bonding capacity makes them ideal for securing fittings and repairing cracked components, contributing to the integrity of buildings.
- Pipes and Tubing: The material’s resistance to stress-cracking and low permeability to gases make it well-suited for plumbing applications.
- Electrical Components: Due to their excellent insulation properties, polyamides are used in electrical fittings, helping to prevent short circuits and electrical fires.
- Concrete Reinforcement: Nylon fibers added to concrete mixtures increase tensile strength and reduce cracking, improving the durability of concrete structures.
Each application capitalizes on the unique features of polyamide polymers, affirming their role as an indispensable ally in the construction industry.
Future Outlook On Polymer of Amino Acids in Construction Applications
The potential of amino acid polymers in construction is vast. As research progresses, biopolymers are expected to play a crucial role in creating eco-friendly materials.
Scientists are exploring the use of amino acid polymers to produce self-healing concrete. This type of concrete can repair its cracks using microorganisms that produce calcium carbonate.
Additionally, bio-based polymeric insulation materials are showing promise for enhanced thermal efficiency and lower environmental impact.
The industry is also witnessing the development of high-performance coatings derived from amino acid polymers that offer better durability and resistance to weathering.
With advancements in synthetic biology and green chemistry, the future may bring construction materials that are not only stronger and more durable but also contribute to a circular economy by being fully recyclable or biodegradable.
Recap




