This informative guide explains what polymers are, how they are made, and their significant role in the construction industry.
Key takeaways:
- Polymers are large, complex molecules made up of repeating structural units called monomers.
- They can be elastic, tough, or biodegradable, and can be molded into various shapes.
- Polymers are durable, lightweight, and resistant to chemicals and weather.
- They have versatile thermal and electrical properties.
- Polymers are used in construction, healthcare, electronics, packaging, automotive, and sports industries.
Definition of Polymer
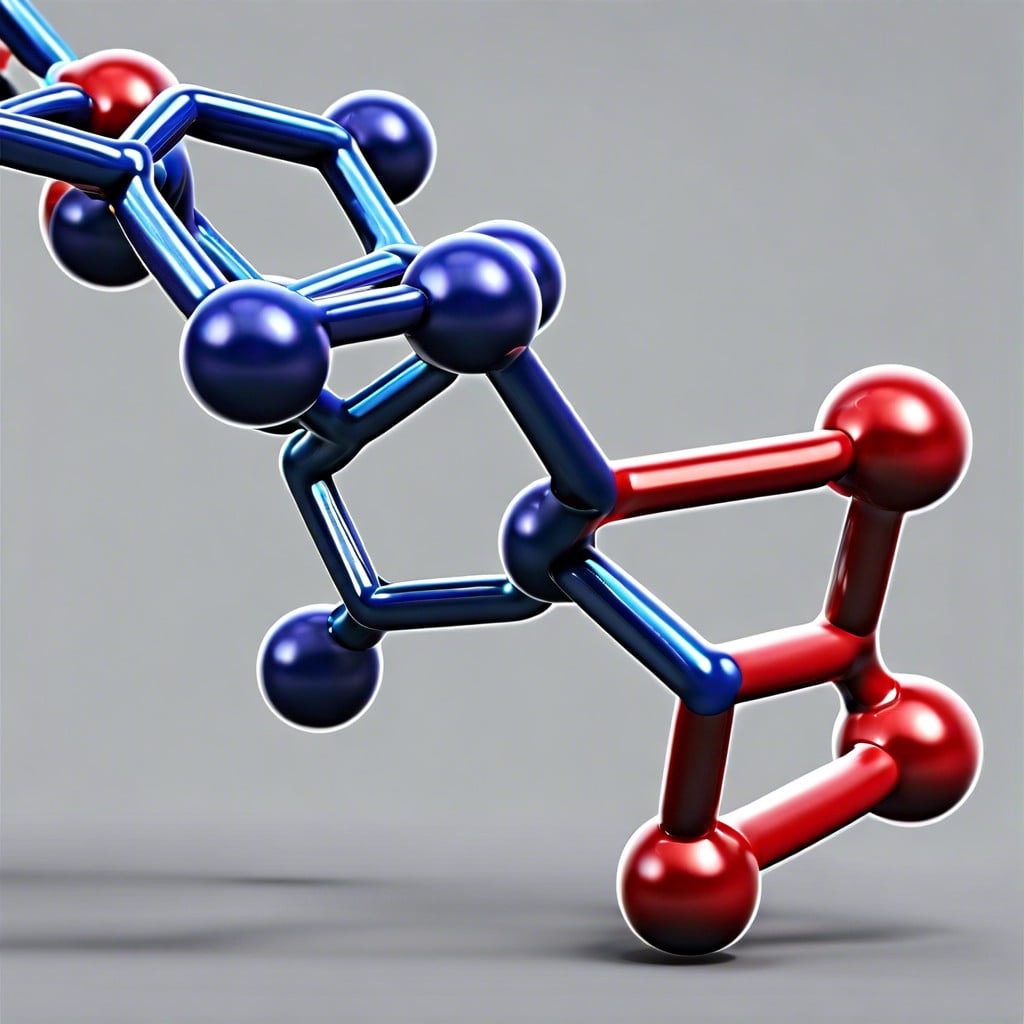
Polymers are large, complex molecules made up of repeating structural units known as monomers. These monomers can be natural, such as glucose in cellulose, or synthetic, like styrene in polystyrene. The bonds that link the monomers together create long chains or networks, contributing to the unique properties of polymers.
In essence, think of a polymer as a chain where each link is a monomer. Just like a chain can be long or short, flexible or rigid, polymers can have varying lengths and can be engineered to be elastic, tough, or even biodegradable. This versatility is due in part to the variety of ways monomers can be combined to form polymers with different structures and functions.
These molecular giants are not static; they can change their state under different conditions. For instance, heating can cause them to soften or melt, while cooling generally makes them harden. This property allows them to be processed and reshaped, fitting various uses from rubber bands to insulation materials.
Characteristics of Polymers
Polymers can exhibit a wide array of qualities that vary depending on their molecular makeup and structure. They are typically durable, lightweight, and resistant to chemicals and weather, which makes them ideal for a multitude of uses, from packaging to construction materials. They also have a unique ability to be molded into almost any shape and can be designed to be either rigid or flexible.
Another prominent characteristic is the versatility in their thermal properties. Some polymers are engineered to withstand extremely high temperatures, while others remain stable at very low temperatures.
Moreover, the electrical insulation properties of polymers are exceptional, which is essential in the electronics industry for coating and protecting wires and components.
Additionally, many polymers have a lower environmental impact in production when compared to materials like metal or glass, due to their lower energy requirements for manufacturing.
The range of mechanical strengths from one polymer to another can be expansive, from soft and stretchy materials like rubber to hard and strong materials akin to Kevlar. This allows polymers to be used across diverse applications demanding distinct mechanical properties.
Classification of Polymers
Polymers are typically sorted into categories based on their origin and thermal characteristics.
Origin-based classifications separate polymers into two groups:
- Natural Polymers: These occur in nature and include proteins, cellulose, and natural rubber.
- Synthetic Polymers: Humans create these in labs and factories, such as plastics, synthetic fibers, and synthetic rubbers.
Thermal properties lead to a different categorization, focusing on how polymers respond to heat:
- Thermosetting Polymers: Once these polymers are heated and formed, they cannot be melted and re-shaped. Examples include epoxy resins and phenolic resins.
- Thermoplastic Polymers: Unlike thermosetting polymers, these can be melted, reshaped, and solidified repeatedly. Common thermoplastics include polyethylene and polystyrene.
Understanding these categories helps in selecting the right type of polymer for specific construction applications, ensuring the material’s performance aligns with the environmental and structural demands of a project.
Polymer Applications in Various Industries
Polymers play a vital role in a myriad of industries due to their diverse properties which can be tailored to meet specific needs.
In construction, they are used for insulation, piping, adhesives, and as structural components in composite materials. Their durability and resistance to elements make buildings safer and more energy-efficient.
The healthcare industry relies on polymers for hygienic coatings, drug delivery systems, and implants that are biocompatible and can withstand sterilization processes.
In electronics, polymers are found in components such as circuit boards, displays, and cables, offering flexibility, electrical insulation, and reduced weight.
Packaging materials frequently use polymers because of their ability to form barriers against moisture, gases, and microbes, thus protecting goods and extending shelf life.
In the automotive sector, the demand for lighter vehicles has increased the use of polymer composites to decrease weight while maintaining strength, aiding in fuel efficiency and reducing emissions.
Sports and leisure goods, including athletic apparel, equipment, and footwear, benefit from polymers for their performance-enhancing characteristics, such as shock absorption, flexibility, and durability.
Environmental and sustainability efforts have spurred the development of biopolymers, derived from renewable sources and designed to be more easily recyclable, thus improving the lifecycle of polymer materials.
Polymer Degradation and Recycling Concerns
Polymers, like other materials, are subject to degradation over time. This process can involve physical wear and tear or chemical breakdown, often accelerated by environmental factors such as UV light, oxygen, and temperature variations. Signs of polymer degradation include discoloration, becoming brittle, or losing structural integrity.
With the vast use of polymers in everyday products, their disposal and recycling present a significant challenge. Not all polymers are easily recyclable, and the processes required can be both energy-intensive and complex. Some common recycling concerns include:
- Separation: Different types of polymers must be meticulously separated before recycling, as mixing can compromise the recycled material’s quality.
- Contamination: Food residue or other contaminants can hinder recycling processes and need to be thoroughly cleaned, increasing the recycling efforts.
- Biodegradability: While some newer polymers are designed to biodegrade, most traditional plastics do not break down easily, contributing to persistent environmental pollution.
- Chemical Recycling: Advanced methods such as chemical recycling can break polymers down to their monomers, but these technologies are still not widespread and can be costly.
To address these concerns, research into developing more sustainable polymers, improving recyclability, and enhancing biodegradation rates are ongoing. Mindful consumption and proper waste management also play crucial roles in lessening the environmental impact of polymer waste.
FAQ
What is polymer in simple words?
A polymer is a large molecule composed of many smaller units linked together, much like a chain.
Is a polymer a plastic?
While all plastics are indeed polymers, it is important to note that not all polymers qualify as plastics.
What is an example of a polymer?
An example of a polymer is vulcanized rubber, which is a synthetic polymer used widely in various industries.
How are polymers used in the construction industry?
Polymers are used in the construction industry as materials for insulation, coatings, sealants, adhesives, foams, and specialty plastics, enhancing durability, resistance to heat, chemicals, and water, and providing thermal and acoustic insulation.
What distinguishes natural polymers from synthetic ones?
Natural polymers occur in nature and are often biodegradable, while synthetic polymers are human-made, typically from petroleum-based products, and often not readily degradable.
How does the structure of a polymer affect its properties?
The structure of a polymer, determined by the type, arrangement and bonding of its monomers, directly influences its properties such as strength, flexibility, chemical resistance, and thermal stability.
Recap




